Introduction
Tiredness and chronic fatigue, loss of strength, cramps, fibromyalgia, arrhythmias, poor digestion and gastric emptying, alternating bowel pattern, mood disorders, irritability and "functional bipolar disorder" are some of the medically-unexplained symptoms (MUS) that our body can show when the transmission of nervous impulses is altered due to ionic concentration changes. In particular, potassium and magnesium alterations are decisive in causing nervous conduction imbalances, with symptoms that affect the muscles, the heart, the nervous and the gastro-intestinal systems.
Potassium is an electrolyte that the body needs in order to carry out a variety of functions, including hydro-electrolytic balance regulation, nervous transmission, conversion of blood sugar into glycogen (Gly) [41] and protein synthesis, while magnesium is indispensable for numerous activities, including enzymatic reactions, maintenance of electrolytic balance, energy metabolism and cell proliferation.
These two minerals are important both individually and together. There is a connection between their concentrations: multiple studies highlight that magnesium deficiency is linked to potassium deficiency [29] and that while isolated potassium balance disorders do not alter magnesium homeostasis, magnesium depletion produces a secondary potassium depletion.
The potassium/magnesium ratio (K/Mg Ratio) (BIA-ACC) is the ratio between these two ions and is a prognostic indicator of the functional capacity of action potentials: the lower than 4.8, the more altered the membrane potential and, as a result, the generation of action potentials in excitable tissues.
It is therefore essential to restore or maintain the proper potassium/magnesium ratio in order to keep the membrane polarization values within their physiological range, thus not altering action potentials - with MUS relating to excitable (muscular, cardiac, gastro-intestinal and nervous) tissues occurring when action potentials change.
Potassium
Potassium is an ion. Most of it (approximately 98%) occurs in intracellular liquids (intra-cellular potassium, ICK) [41], with the remaining 2% being in extracellular liquids (Extra-cellular potassium, ECK) [41]. The same allocation applies to the sodium-potassium pump, that is of fundamental importance for the passage of molecules against the electrochemical and concentration gradient through the cell membrane. A different intra- and extra-cellular concentration gradient is necessary for cell polarization, which affects multiple processes such as nervous impulses and muscle cell contraction (including the cardiac muscle). As such, relatively small changes in the serum concentration of this ion can have significant clinical manifestations. In adults, the minimum value of total body potassium (total body potassium - TBK) [41] is approximately 2500 mmol.
Inside cells, potassium is necessary for normal cell growth and protein synthesis. As most intracellular potassium is contained inside muscle cells, total body potassium is pro-rated to the body fat-free mass [1] and in particular to the skeletal muscle (Skeletal Muscle FFM) [41].
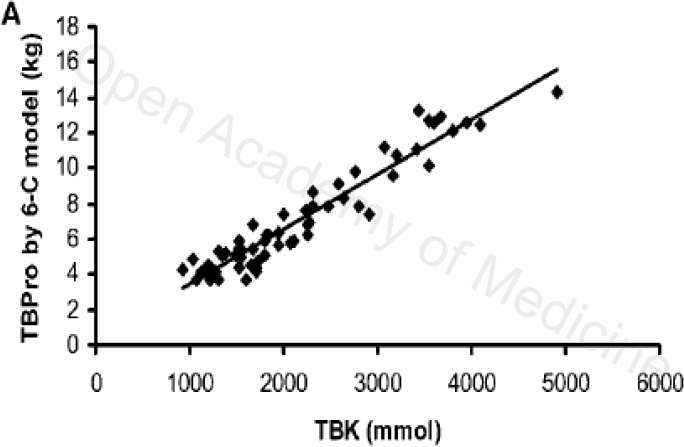
Figure 1: JN THE JOURNAL OF NUTRITION
This chart shows a linearity between total body potassium (TBK) [41] and total body proteins (Tbprotein) [41]: the higher the TBprotein, the higher total body potassium. Clearly, the causes of potassium loss are linked to all those situations in which there is a loss of fat-free mass (“starvation”, sarcopenia, cachexia, sedentary lifestyle, inflammatory diseases, kidney and bowel diseases and cancer).
Extracellular potassium and action potential
Extracellular potassium is of fundamental importance for maintaining the efficiency of the sodium-potassium pump, which serves to preserve the electrical charge inside the cell (a particularly important function for nervous muscle cells).
It plays a central role
Potassium loss from the intracellular to the extracellular environment (ECK) [41] leads to altered cell membrane potential polarization, lower tissue excitability and altered function of the neuromuscular, cardiac and gastrointestinal systems, with the consequent occurrence of medically-unexplained symptoms (MUS) in a range of systems:
In addition, potassium is essential for converting blood sugar into glycogen (Gly) i.e. the form in which glucose is stored in the muscles and the liver (gly or glycogen mass) [41]. Reduced glycogen storage causes an increase in fatigue and muscle weakness [3] and an increase in free glycogen (Gly Free – free glycogen) [41] leading to lipogenesis and hence to an increase of adipose tissue; in particular, this results in abdominal and visceral fat buildup [39].
Action potential alterations
This figure shows the action potential with different intra- and extra-cellular potassium ratios. Changes in the intra- and extra-cellular potassium ratio cause a shift in the resting membrane potential (REMP) and, overall, tend to prolong the action potential due to the longer late re-polarization phase mainly in relation to potassium currents. Intra-cellular potassium loss, through a disruption of the Ki/Ke balance, leads to cellular hyperpolarization, longer time of activation and cellular depolarization. In principle, the hyperpolarization of a single cell type should not generate arrhythmias because it implies that the REMP shifts away from the activation potential but in fact, hyperpolarization encourages re-entry phenomena. Different cell types (nodal, conduction and muscular) coexist in the heart and the different polarization of cellular elements creates dyssynchrony and triggers a mechanism that underlies the genesis of re-entry phenomena. Hence, a reduction in intra-cellular potassium is an arrhythmogenic risk factor, as confirmed by a study demonstrating that the intra-erythrocyte potassium of hemodialysis patients with arrhythmias was lower compared to non-arrhythmic patients. More recently, it has been reported that a significant loss of potassium occurring during standard hemodiafiltration affects the cell electric potentials, determining a greater risk of arrhythmias in patients treated with hemodialysis [43].
Indeed, the heart’s electrical stability is more sensitive to the concentration of extracellular potassium (ECK - extracellular potassium) [41] compared to intra-cellular potassium: an ischemic myocardium loses potassium in the extracellular space in a matter of few seconds and the cell becomes depolarized [40].
Changes in extracellular potassium can also occur in normal conditions, due to
the phenomenon that occurs is similar to the one described above: the resting membrane potential, which in normal conditions is -84 mV, increases and can reach approximately -67 mV. Still, when trans-membrane potentials exceed -70 mV, the sodium inactivation channels are closed, thus making the tissue not excitable [14]. For this reason, an increase in extracellular potassium (ECK) [41], alters the stimulation threshold as well as the excitation mechanism; this condition seems to be due to the opening of dependent ATP channels [22,23,42].
Magnesium
Magnesium is the second most abundant intracellular cation. Approximately half of the total body magnesium (TBMg - total body magnesium) [41] occurs in soft tissues (Stm - soft tissue minerals) [41], with the remaining half being in bones (approximately 60%) and less than 1% in extracellular liquids (this concentration is important for the transmission of nervous impulses and for the control of muscular contractions). Magnesium is indispensable for numerous enzymatic activities. Indeed, it is involved in the regulation mechanisms of 300 different enzymatic complexes, is important for maintaining the electrolytic balance and is of fundamental importance for normal neuromuscular function as well as for the transport of calcium and potassium, besides being necessary for activating the potassium pump, energy metabolism and cell proliferation [12, 44].
Absorption
It is absorbed by the small intestine and its serum concentration is controlled through renal excretion.
Magnesium deficiency is extremely common and linked to a number of factors that reduce the absorption or increase the secretion of magnesium; the causes can be linked to
the most common symptoms of magnesium deficiency are:
Magnesium deficiency and diseases
As a result of low levels of magnesium, the body is more prone to diseases such as heart diseases, arterial hypertension, kidney stones and depression. A study carried out in the United States on individuals with magnesium deficiency has shown that these individuals were more prone to diseases such as atherosclerosis, myocardial infarction, hypertension, cancer, kidney stones, premenstrual syndrome and psychiatric disorders [4].
Other research studies focused on demonstrating the efficacy of magnesium in treating arrhythmias, severe asthma and migraine; its use for constipation and dyspepsia is an accepted standard therapy, despite there being limited evidence [2,3,5,6,7]. For this reason, an increased intake of magnesium can benefit people with:
Magnesium and action potential
In the cardiac muscles, magnesium depletion can produce acute electrocardiographic changes. As a matter of fact, hypomagnesemia plays a role in severe ventricular arrhythmia [10]. Studies show that this ion has a depressive action on the heart: an induced increase in the concentration of magnesium ions determines a reduction in the sinus rate.
In-vivo experiments have shown that increased magnesium concentration causes hypotension due to vasodilation, whereas its decrease is often associated to increased blood pressure caused by vasoconstriction. This data suggests that magnesium can impact the excitation-contraction coupling in vascular smooth muscles. Studies have been conducted on the effects of higher extra-cellular magnesium concentration on the mechanical and electrophysiological properties of smooth muscles, with higher extra-cellular magnesium reducing the width of both electrically-induced and spontaneous contractions. A higher magnesium concentration leads to a regulation of the calcium supply through voltage-dependent channels, thus regulating muscular contractility [11].
Importance of Magnesium for controlling Potassium (K/Mg Ratio)
These two minerals are important both individually and together. Indeed, there is a connection between their concentrations. Evidence suggest that magnesium deficiency is linked to potassium deficiency [29]: hypomagnesemia is linked to the onset of hypokalemia and hypocalcemia. Hypokalemia is common in patients with hypomagnesemia and occurs in 40-60% of cases. This seems to result from the inability of cells to maintain the normal intercellular concentration of potassium, possibly due to increased permeability of the membrane to potassium and/or inhibition of Na-K ATPase. As a result, cells lose potassium, which is secreted in the urine. Potassium depletion in cells requires a correction of magnesium deficiency.
Isolated potassium balance disorders do not produce alterations of magnesium homeostasis. Instead, magnesium depletion produces a secondary potassium depletion.
With BIA-ACC it is possible to evaluate the levels of extracellular potassium, magnesium and the K/Mg Ratio (that is to say, the ratio between these two ions).
The K/Mg Ratio is a prognostic indicator of the functional capacity of action potentials; its ideal value is 4.8 (normal value: 4.6-5) and represents the maximum activation of action potentials (see supplements with a proper Mg/K Ratio). Maintenance of the K/Mg Ratio is fundamental for
The lower this value, the higher the perception of medically-unexplained symptoms (MUS): a linear loss of the K/Mg Ratio with values lower than 4.5 leads to widespread medically-unexplained symptoms (MUS) in relation to excitable tissues, with an impact on the following apparatuses:
Magnesium and Potassium: fields of application
Potassium and muscle fatigue
As described above, potassium is essential for converting blood sugar into glycogen (Gly) [41]. Glycogen storage reduction causes an increase in fatigue and muscle weakness.
Cardiovascular: hypertension
Potassium and magnesium are useful in cardiovascular disorders: the potential of high-potassium diets to lower blood pressure results, at least in part, from the natriuretic activity of the ion that promotes the renal excretion of sodium and decreases the adverse impact of high-sodium diets. Besides, the modest increase in serum potassium that is achievable with a high-potassium diet affects the hyperpolarization of the vascular endothelium and ultimately increases the production of endothelial nitric oxide while suppressing the production of superoxide. Epidemiological studies show that increased potassium intake can reduce the risk of infarction and above all stroke [13].
Other research studies show a reverse relationship between arterial pressure and potassium intake and this effect seems to be higher in people with hypertension as compared to those with in-range blood pressure levels [14].
Magnesium as well seems to be useful in treating hypertension: small changes in magnesium levels can significantly affect cardiac excitability, vascular tone, contractility and reactivity of the heart. Most epidemiological and experimental studies have shown a reverse association between magnesium concentration and blood pressure, so much so that magnesium supplementation is advisable for hypertense patients on diuretics with resistant or secondary hypertension [8,15].
Another study shows that the degree of intra-cellular magnesium deficiency in women with angina is closely related to the frequency of chest pain [16].
These benefits add to the considerations made on the importance of these two minerals in modulating action potentials.
Neurological problems
Magnesium plays a major role in the conduction of the Nervous System. Magnesium deficiency is a well-known risk factor for the development of neuropathies, including depression. It has been shown that magnesium intake enables quick (less than 7 days) recovery from depressive symptoms. Taking this ion has also been beneficial for individuals with cranial traumas, headache, suicidal ideation, anxiety, irritability, insomnia, post-partum depression and short-term memory loss [17]. The disorders due to magnesium deficiency can be explained by the fact that it opens the N- methyl-D-aspartate (NMDA) calcium channels, thus causing neuronal damage and neurological disfunctions such as major depression. The effects of the oral administration of magnesium compare to those of anti-depressants [8,9,18].
Also the most common states of anxiety are linked to magnesium deficit: there is a correlation between disrupted magnesium homeostasis and pathological anxiety. Magnesium deficiency causes an increase in the hormone that releases corticotropin in the paraventricular hypothalamic nucleus, which, in turn, leads to higher ACTH, thus indicating a greater stimulation of the HPA axis.
Recent research has shown that seizures often coincide with a higher concentration of extracellular potassium: experimental findings have revealed a high concentration of extracellular potassium during seizure attacks [19].
Based on the above, the studies relating to the effects of a high extra-cellular concentration of potassium on the hippocampus interneurons have found that a higher concentration was followed by higher discharge activity, indicating that the increase in potassium observed during the attack stimulated inter-neuronal activities and suggested a loss or an impairment of the neuronal inhibitory function [20].
In addition, it seems that high extracellular potassium can contribute to the physiopathology of major neurological disorders, including cerebral ischemia and migraine [21].
Gastro-intestinal disorders
A magnesium-deficient diet also impacts the gastro-intestinal tract, with resulting alterations in the motility of the digestive tube, possibly leading to colon atony. Magnesium deficiency can induce a significant increase in intestinal inflammation (as evaluated based on the infiltration of neutrophils) and cause significant functional changes in local organs and a higher sensitivity to oxidative stress.
Magnesium supplementation is useful as it increases the release of cholecystokinin (CCK), leading to a buildup of intestinal fluid and stimulating the motility of the small intestine [25,26,27,28].
Conclusions
Magnesium is essential [30,31,32,33,34,35,36] for
Likewise, for the homeostasis of calcium and potassium, magnesium deficiency is linked to potassium and calcium deficiency. For this reason, magnesium supplementation is also useful for the health of bones and teeth.
In turn, potassium plays a role in [37,38]:
Clearly, a combined supplementation of these two ions benefits all excitable (muscular, cardiovascular, nervous and gastrointestinal) tissues in our body.
Besides, these two ions are important both individually and together: the K/Mg Ratio provides an indicative value of the activation of the action potential of excitable tissues and the lower this ratio compared to ideal value (4.8) (normal value: 4,6-5) the higher the perception of medically-unexplained symptoms (MUS).
In addition, one should be mindful that potassium depends on magnesium, as magnesium deficiency results in secondary potassium deficiency whereas potassium deficiency is not linked to magnesium deficiency.
Magnesium and potassium food supplements (ideally taken at mid-morning, before a snack) with a magnesium-to-potassium ratio of (1:4.8) have been evaluated instrumentally by measuring the recovery of total body potassium (TBK) [41] and extra-cellular potassium (ECK) with the primary aim to stabilize extra-cellular potassium at a maximum value of 2% of total potassium, increasing the cellular efficiency of excitable tissues. This results in a higher number of activations of the action potential. More frequent action potentials lead to more central and peripheral nervous system feedback, with a physiological activation of the neuro-vegetative system (NVS).
Therefore, activating action potentials that, for a variety of reasons, have been altered due to depolarization, considerably benefits all excitable (muscular, cardiac, gastro-intestinal and nervous) tissues.
Author: Dario Boschiero - Date: 29/06/2021
Attention: these contents can be freely used for personal learning purposes only. The use is regulated by Law No. 633/1941 and subsequent amendments, as well as by the copyright and patent legislation in force. Any use for commercial and profit-making purposes is forbidden.
References