Vague and non-specific symptoms (Medically Unexplained Symptoms, MUS)
In most of the examinations carried out at General Medicine outpatient clinics, physicians focus their attention on overt clinical data, characterized by clearly recognizable symptoms. Other symptoms, which could be defined as vague, such as chronic fatigue, sleep or appetite disorders, irritable bowel or constipation, tone or mood disorders, non-specific pain syndromes etc., are sometimes underestimated. These signs remain largely unexplained, i.e. they are not traced back to a precise cause, disease or disorder. In Anglo-Saxon literature they are identified as “MUS”, Medically Unexplained Symptoms. The appearance of MUS in general medicine patients is becoming increasingly frequent, and on several occasions [1, 2, 11, 14, 17, 21] it has been pointed out how difficult it is to treat these symptoms, both from a diagnostic and, consequently, from a therapeutic standpoint. Therefore, it is important to emphasize the risk that a psychosocial diagnosis may imply: due to the objective difficulties related to medical investigation, hidden diseases may be overlooked [17]. Most of the publications on this subject describe a situation whose complexity starts from the interview with the patient, who can hardly communicate his own discomforts and symptoms in a precise way. The various traditional classification tools, based on patient interviews [12], do not seem to be particularly convincing. Due to the lack of concretely measurable data, they can hardly escape the patient’s subjective perception. Significant progress in the assessment of MUS and their impact can be achieved if such interviews are complemented by precise tools that provide objective measurements (see TomEEx - Extracellular Electrolyte Tomography, BIA-ACC - Advanced Clinical Body Composition Analysis and PPG Stress Flow - Analysis, monitoring and biofeedback of the autonomic nervous system and of heart rate variability) of parameters showing the patient’s health.
The role of HPA axis and chronic inflammation
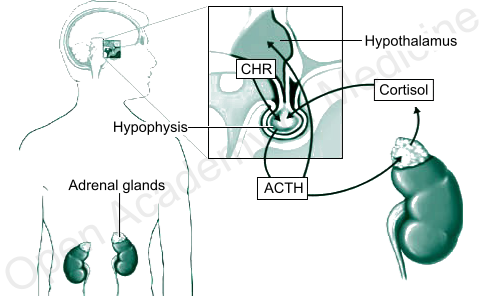
![]() The circadian rhythm of cortisol secretion is an important endogenous synchronization factor for the human body, and it should be harmonized with the current receptive state of cells as well as with the needs of the organism. In a healthy person, CRH (hypothalamus) and ACTH (pituitary gland) secretions are particularly sensitive to the decrease in cortisol levels during nighttime [36], so as to trigger the acrophase (peak concentration time) of cortisol circadian rhythm about half an hour after waking up, thus promoting the body’s stress response and adaptation processes. The increase in cortisol, once identified by the pituitary gland, causes in turn a progressive drop in ACTH secretion (retroactive HPA regulation) and thus a subsequent reduction in the activity of the adrenal glands and in cortisol secretion. However, a change in the circadian rhythm of glucocorticoids and in particular an abnormal flattening of the cortisol curve (i.e., when its secretion persists over time), due to a condition of chronic stress of the HPA axis (HPA axis index - BIA-ACC), is related to the onset of many diseases, hence the need for a convenient tool that can assess the reaction and feedback ability of the HPA axis (see TomEEx - Extracellular Electrolyte Tomography and BIA-ACC - Advanced Clinical Analysis of Body Composition). There are numerous studies on the relationships between various diseases and HPA axis imbalances, clearly showing links between abnormal glucocorticoid levels and the most varied diseases, such as depression [2, 12], anxiety and panic attacks [5, 33, 38], hippocampal hypofunction and impaired memory capacities [6], sleep disorders [6, 7, 9, 19, 27], chronic fatigue syndrome (CFS) [24], fibromyalgia and autoimmune diseases [10, 31], irritable bowel syndrome [23], hypertension [35], eating disorders and obesity [8, 18, 25, 26, 30, 32], rheumatism [4, 20] etc. The persistent activation of the HPA axis (HPA axis index: Flat Low/High - BIA-ACC) can in turn be caused by the chronicity of inflammatory processes [28, 29]. In such cases, however, the anti-inflammatory action of glucocorticoids will rapidly decline, also due to the imbalance vis-à-vis the receptivity of cells, while the increase in glucocorticoid levels and the alteration of their circadian rhythms will continue to stress the body.
The circadian rhythm of cortisol secretion is an important endogenous synchronization factor for the human body, and it should be harmonized with the current receptive state of cells as well as with the needs of the organism. In a healthy person, CRH (hypothalamus) and ACTH (pituitary gland) secretions are particularly sensitive to the decrease in cortisol levels during nighttime [36], so as to trigger the acrophase (peak concentration time) of cortisol circadian rhythm about half an hour after waking up, thus promoting the body’s stress response and adaptation processes. The increase in cortisol, once identified by the pituitary gland, causes in turn a progressive drop in ACTH secretion (retroactive HPA regulation) and thus a subsequent reduction in the activity of the adrenal glands and in cortisol secretion. However, a change in the circadian rhythm of glucocorticoids and in particular an abnormal flattening of the cortisol curve (i.e., when its secretion persists over time), due to a condition of chronic stress of the HPA axis (HPA axis index - BIA-ACC), is related to the onset of many diseases, hence the need for a convenient tool that can assess the reaction and feedback ability of the HPA axis (see TomEEx - Extracellular Electrolyte Tomography and BIA-ACC - Advanced Clinical Analysis of Body Composition). There are numerous studies on the relationships between various diseases and HPA axis imbalances, clearly showing links between abnormal glucocorticoid levels and the most varied diseases, such as depression [2, 12], anxiety and panic attacks [5, 33, 38], hippocampal hypofunction and impaired memory capacities [6], sleep disorders [6, 7, 9, 19, 27], chronic fatigue syndrome (CFS) [24], fibromyalgia and autoimmune diseases [10, 31], irritable bowel syndrome [23], hypertension [35], eating disorders and obesity [8, 18, 25, 26, 30, 32], rheumatism [4, 20] etc. The persistent activation of the HPA axis (HPA axis index: Flat Low/High - BIA-ACC) can in turn be caused by the chronicity of inflammatory processes [28, 29]. In such cases, however, the anti-inflammatory action of glucocorticoids will rapidly decline, also due to the imbalance vis-à-vis the receptivity of cells, while the increase in glucocorticoid levels and the alteration of their circadian rhythms will continue to stress the body.
![]()
From a hydroelectrolytic standpoint, chronic inflammation is locally characterized by an increase in extracellular electrolytes and a decrease in intracellular electrolytes and, at a systemic level, by dehydration and a loss of electrolytes that are recruited to the inflamed sites as they help keep the extracellular pH stable. If persistent over time, this systemic loss leads to lower electrolyte reserves, and in particular to lower phosphate and bicarbonate buffers (see Insights on extracellular tissue pH, oxidative stress and clinical observations, BIA-ACC). If persistent, the ionic decompensation in the inflamed regions leads to an alteration of the membrane potential (caused by the difference in electrolyte concentration inside and outside the cell), which can go as far as polarity reversal.
Hydroelectrolyte regulation in the presence of chronic inflammation
In order to restore the HPA axis physiological condition, it is necessary to regulate the ionic balance in chronically inflamed regions, so as to bring glucocorticoid secretion back to normal and thus restore the physiological circadian rhythms. The areas affected by chronic inflammatory processes are characterized by a high level of ionic exchange, and therefore by a current flow that is so strong as to generate an electromagnetic field, which can be detected by the biofeedback therapeutic device RegMatEx - Extracellular Matrix Regulation. Based on the spectrum analysis of the acquired signal, the device performs – after specific correction procedures (potentials, frequencies and magnitude) – a selective electromagnetic stimulation of “ionic compensation” to rebalance the level of intra-extracellular exchange in the chronically inflamed regions and thus reduce the level of inflammation by lowering the concentration of glucocorticoids. To support the device function, it is of course necessary to ensure adequate hydration and prior availability of basic electrolytes, such as bisphosphates, bicarbonates, magnesium, potassium and calcium, at a systemic level (see insights on tissue pH and oxidative stress and treatment preparation, RegMatEx).
Author: Dario Boschiero - Date: 13/11/2020
Attention: these contents can be freely used for personal learning purposes only. The use is regulated by Law No. 633/1941 and subsequent amendments, as well as by the copyright and patent legislation in force. Any use for commercial and profit-making purposes is forbidden.
References
- Epstein RM, Shields CG, Meldrum SC, Fiscella K, Carroll J, Carney PA, Duberstein PR, Physicians' responses to patients' medically unexplained symptoms, Psychosom Med, 2006 Mar-Apr, 68(2):269-76;
- Keller J, Flores B, Gomez RG, Solvason HB, Kenna H, Williams GH, Schatzberg AF, Cortisol Circadian Rhythm Alterations in Psychotic Major Depression, Biol Psychiatry, 2006 Feb 1;
- Ringsberg KC, Krantz G, Coping with patients with medically unexplained symptoms: work-related strategies of physicians in primary health care, J Health Psychol, 2006 Jan, 11(1):107-16;
- Cutolo M, Villaggio B, Otsa K, Aakre O, Sulli A, Seriolo B, Altered circadian rhythms in rheumatoid arthritis patients play a role in the disease's symptoms, Autoimmun Rev. 2005 Nov, 4(8):497-502;
- Takahashi T, Ikeda K, Ishikawa M, Kitamura N, Tsukasaki T, Nakama D, Kameda T, Anxiety, reactivity, and social stress-induced cortisol elevation in humans, Neuro Endocrinol Lett, 2005 Aug, 26(4):351-4;
- Buckley TM, Schatzberg AF, Aging and the role of the HPA axis and rhythm in sleep and memory-consolidation, Am J Geriatr Psychiatry, 2005 May, 13(5):344-52;
- Buckley TM, Schatzberg AF, On the interactions of the hypothalamic-pituitary-adrenal (HPA) axis and sleep: normal HPA axis activity and circadian rhythm, exemplary sleep disorders, J Clin Endocrinol Metab, 2005 May, 90(5):3106-14;
- Gluck ME, Geliebter A, Hung J, Yahav E, Cortisol, hunger, and desire to binge eat following a cold stress test in obese women with binge eating disorder, Psychosom Med, 2004 Nov-Dec;66(6):876-81;
- Backhaus J, Junghanns K, Hohagen F, Sleep disturbances are correlated with decreased morning awakening salivary cortisol, Psychoneuroendocrinology, 2004 Oct, 29(9):1184-91;
- Crofford LJ, Young EA, Engleberg NC, Korszun A, Brucksch CB, McClure LA, Brown MB, Demitrack MA, Basal circadian and pulsatile ACTH and cortisol secretion in patients with fibromyalgia and/or chronic fatigue syndrome, Brain Behav Immun, 2004 Jul, 18(4):314-25;
- Woivalin T, Krantz G, Mantyranta T, Ringsberg KC, Medically unexplained symptoms: perceptions of physicians in primary health care, Fam Pract, 2004 Apr, 21(2):199-203;
- Smith RC, Korban E, Kanj M, Haddad R, Lyles JS, Lein C, Gardiner JC, Hodges A, Dwamena FC, Coffey J, Collins C, A method for rating charts to identify and classify patients with medically unexplained symptoms, Psychother Psychosom, 2004 Jan-Feb;73(1):36-42;
- Mello Ade A, Mello MF, Carpenter LL, Price LH, Update on stress and depression: the role of the hypothalamic-pituitary-adrenal (HPA) axis, Rev Bras Psiquiatr, 2003 Oct, 25(4):231-8;
- Smith RC, Lein C, Collins C, Lyles JS, Given B, Dwamena FC, Coffey J, Hodges A, Gardiner JC, Goddeeris J, Given CW, Treating patients with medically unexplained symptoms in primary care, J Gen Intern Med, 2003 Jun, 18(6):478-89;
- Chan O, Inouye K, Riddell MC, Vranic M, Matthews SG, Diabetes and the hypothalamo-pituitary-adrenal (HPA) axis, Minerva Endocrinol, 2003 Jun;28(2):87-102;
- Gaillard RC, [Interactions between the immune and neuroendocrine systems: clinical implications], J Soc Biol, 2003, 197(2):89-95;
- Albrecht S, Naugle AE, Psychological assessment and treatment of somatization: adolescents with medically unexplained neurologic symptoms, Adolesc Med, 2002 Oct, 13(3):625-41;
- Vicennati V, Ceroni L, Gagliardi L, Gambineri A, Pasquali R, Comment: response of the hypothalamic-pituitary-adrenocortical axis to high-protein/fat and high-carbohydrate meals in women with different obesity phenotypes, J Clin Endocrinol Metab, 2002 Aug, 87(8):3984-8;
- Rodenbeck A, Huether G, Ruther E, Hajak G, Interactions between evening and nocturnal cortisol secretion and sleep parameters in patients with severe chronic primary insomnia, Neurosci Lett, 2002 May 17;324(2):159-63;
- Crofford LJ, The hypothalamic-pituitary-adrenal axis in the pathogenesis of rheumatic diseases, Endocrinol Metab Clin North Am, 2002 Mar, 31(1):1-13;
- Reid S, Whooley D, Crayford T, Hotopf M, Medically unexplained symptoms--GPs' attitudes towards their cause and management, Fam Pract, 2001 Oct, 18(5):519-23;
- Gaillard RC, Interaction between the hypothalamo-pituitary-adrenal axis and the immunological system, Ann Endocrinol (Paris), 2001 Apr, 62(2):155-63;
- Elsenbruch S, Orr WC, Diarrhea and constipation-predominant IBS patients differ in postprandial autonomic and cortisol responses, Am J Gastroenterol, 2001 Feb, 96(2):460-6;
- Racciatti D, Guagnano MT, Vecchiet J, De Remigis PL, Pizzigallo E, Della Vecchia R, Di Sciascio T, Merlitti D, Sensi S, Chronic fatigue syndrome: circadian rhythm and hypothalamic-pituitary-adrenal (HPA) axis impairment, Int J Immunopathol Pharmacol, 2001 Jan, 14(1):11-15;
- Epel E, Lapidus R, McEwen B, Brownell K, Stress may add bite to appetite in women: a laboratory study of stress-induced cortisol and eating behavior, Psychoneuroendocrinology, 2001 Jan, 26(1):37-49;
- Vicennati V, Pasquali R, Abnormalities of the hypothalamic-pituitary-adrenal axis in nondepressed women with abdominal obesity and relations with insulin resistance: evidence for a central and a peripheral alteration, J Clin Endocrinol Metab, 2000 Nov, 85(11):4093-8;
- Blazejova K, Nevsimalova S, Illnerova H, Hajek I, Sonka K, [Sleep disorders and the 24-hour profile of melatonin and cortisol], Sb Lek, 2000, 101(4):347-51;
- Harbuz MS, Chronic inflammatory stress, Baillieres Best Pract Res Clin Endocrinol Metab, 1999 Dec, 13(4):555-65;
- Shanks N, Harbuz MS, Jessop DS, Perks P, Moore PM, Lightman SL, Inflammatory disease as chronic stress, Ann N Y Acad Sci, 1998 May 1, 840:599-607;
- Leal AM, Moreira AC, Food and the circadian activity of the hypothalamic-pituitary-adrenal axis, Braz J Med Biol Res, 1997 Dec, 30(12):1391-405;
- Harbuz MS, Conde GL, Marti O, Lightman SL, Jessop DS, The hypothalamic-pituitary-adrenal axis in autoimmunity, Ann N Y Acad Sci, 1997 Aug 14, 823:214-24;
- Van Cauter EV, Polonsky KS, Blackman JD, Roland D, Sturis J, Byrne MM, Scheen AJ, Abnormal temporal patterns of glucose tolerance in obesity: relationship to sleep-related growth hormone secretion and circadian cortisol rhythmicity, J Clin Endocrinol Metab, 1994 Dec, 79(6):1797-805;
- Yehuda R, Boisoneau D, Mason JW, Giller EL, Glucocorticoid receptor number and cortisol excretion in mood, anxiety, and psychotic disorders, Biol Psychiatry, 1993 Jul 1-15, 34(1-2):18-25;
- Tsigos C, Young RJ, White A, Diabetic neuropathy is associated with increased activity of the hypothalamic-pituitary-adrenal axis, J Clin Endocrinol Metab, 1993 Mar, 76(3):554-8;
- Mantero F, Boscaro M, Glucocorticoid-dependent hypertension, J Steroid Biochem Mol Biol, 1992 Oct, 43(5):409-13;
- Angeli A, Glucocorticoid secretion: a circadian synchronizer of the human temporal structure, J Steroid Biochem, 1983 Jul, 19(1B):545-54;
- Zhurova MV, Lugovaia NA, [Carbohydrate tolerance and islet apparatus function in patients with different forms of hypothyroidism], Probl Endokrinol (Mosk), 1983 May-Jun, 29(3):36-40;
- Curtis GC, Nesse R, Buxton M, Lippman D, Anxiety and plasma cortisol at the crest of the circadian cycle: reappraisal of a classical hypothesis, Psychosom Med, 1978 Aug, 40(5):368-78.
 The circadian rhythm of cortisol secretion is an important endogenous synchronization factor for the human body, and it should be harmonized with the current receptive state of cells as well as with the needs of the organism. In a healthy person, CRH (hypothalamus) and ACTH (pituitary gland) secretions are particularly sensitive to the decrease in cortisol levels during nighttime [36], so as to trigger the acrophase (peak concentration time) of cortisol circadian rhythm about half an hour after waking up, thus promoting the body’s stress response and adaptation processes. The increase in cortisol, once identified by the pituitary gland, causes in turn a progressive drop in ACTH secretion (retroactive HPA regulation) and thus a subsequent reduction in the activity of the adrenal glands and in cortisol secretion. However, a change in the circadian rhythm of glucocorticoids and in particular an abnormal flattening of the cortisol curve (i.e., when its secretion persists over time), due to a condition of chronic stress of the HPA axis (HPA axis index - BIA-ACC), is related to the onset of many diseases, hence the need for a convenient tool that can assess the reaction and feedback ability of the HPA axis (see TomEEx - Extracellular Electrolyte Tomography and BIA-ACC - Advanced Clinical Analysis of Body Composition). There are numerous studies on the relationships between various diseases and HPA axis imbalances, clearly showing links between abnormal glucocorticoid levels and the most varied diseases, such as depression [2, 12], anxiety and panic attacks [5, 33, 38], hippocampal hypofunction and impaired memory capacities [6], sleep disorders [6, 7, 9, 19, 27], chronic fatigue syndrome (CFS) [24], fibromyalgia and autoimmune diseases [10, 31], irritable bowel syndrome [23], hypertension [35], eating disorders and obesity [8, 18, 25, 26, 30, 32], rheumatism [4, 20] etc. The persistent activation of the HPA axis (HPA axis index: Flat Low/High - BIA-ACC) can in turn be caused by the chronicity of inflammatory processes [28, 29]. In such cases, however, the anti-inflammatory action of glucocorticoids will rapidly decline, also due to the imbalance vis-à-vis the receptivity of cells, while the increase in glucocorticoid levels and the alteration of their circadian rhythms will continue to stress the body.
The circadian rhythm of cortisol secretion is an important endogenous synchronization factor for the human body, and it should be harmonized with the current receptive state of cells as well as with the needs of the organism. In a healthy person, CRH (hypothalamus) and ACTH (pituitary gland) secretions are particularly sensitive to the decrease in cortisol levels during nighttime [36], so as to trigger the acrophase (peak concentration time) of cortisol circadian rhythm about half an hour after waking up, thus promoting the body’s stress response and adaptation processes. The increase in cortisol, once identified by the pituitary gland, causes in turn a progressive drop in ACTH secretion (retroactive HPA regulation) and thus a subsequent reduction in the activity of the adrenal glands and in cortisol secretion. However, a change in the circadian rhythm of glucocorticoids and in particular an abnormal flattening of the cortisol curve (i.e., when its secretion persists over time), due to a condition of chronic stress of the HPA axis (HPA axis index - BIA-ACC), is related to the onset of many diseases, hence the need for a convenient tool that can assess the reaction and feedback ability of the HPA axis (see TomEEx - Extracellular Electrolyte Tomography and BIA-ACC - Advanced Clinical Analysis of Body Composition). There are numerous studies on the relationships between various diseases and HPA axis imbalances, clearly showing links between abnormal glucocorticoid levels and the most varied diseases, such as depression [2, 12], anxiety and panic attacks [5, 33, 38], hippocampal hypofunction and impaired memory capacities [6], sleep disorders [6, 7, 9, 19, 27], chronic fatigue syndrome (CFS) [24], fibromyalgia and autoimmune diseases [10, 31], irritable bowel syndrome [23], hypertension [35], eating disorders and obesity [8, 18, 25, 26, 30, 32], rheumatism [4, 20] etc. The persistent activation of the HPA axis (HPA axis index: Flat Low/High - BIA-ACC) can in turn be caused by the chronicity of inflammatory processes [28, 29]. In such cases, however, the anti-inflammatory action of glucocorticoids will rapidly decline, also due to the imbalance vis-à-vis the receptivity of cells, while the increase in glucocorticoid levels and the alteration of their circadian rhythms will continue to stress the body.